An Experienced Support in the Development of your Healthand Bio-Engineering Products

Mustapha Belgsir, PhD is the founder of Pharmeb. He is a bioorganic chemist. He held a research position at the CNRS from 1990 to 2002, during which he was also a consultant to chemical industries and an expert to the European Commission.Then he founded and managed BioCydex from 2002 to 2024.
BioCydex was a partner trusted by leading pharma groups, startups and institutional organizations for its unique skills in Pharmaceutical Organic and Physical Chemistry, Analytical Chemistry (Chromatography, Mass Spectrometry, NMR, spectroscopy...) and elementary in vitro tests (toxicity, permeability, hemolytic activity...).
With decades of experience in Drug and Drug Delivery developments, Pharmeb-Consulting implements its skills to support you from early-stage evaluation through to preparing successful manufacturing of drug substances and drug products under pharmacopoeia standards.
Pharmeb-Consulting offers scientific,strategic and monitoring support forthe development of your projects
Pharmeb has established a network of partner platforms to meet your needs during early-stage development of your products:
- custom synthesis of APIs
- characterization and synthesis of impurities (eg. nitrosamines), degradation products, analytical standards (stable isotopes) and metabolites
- solubilization of hydrophobic APIs
- formulation
- qualification of analytical methods
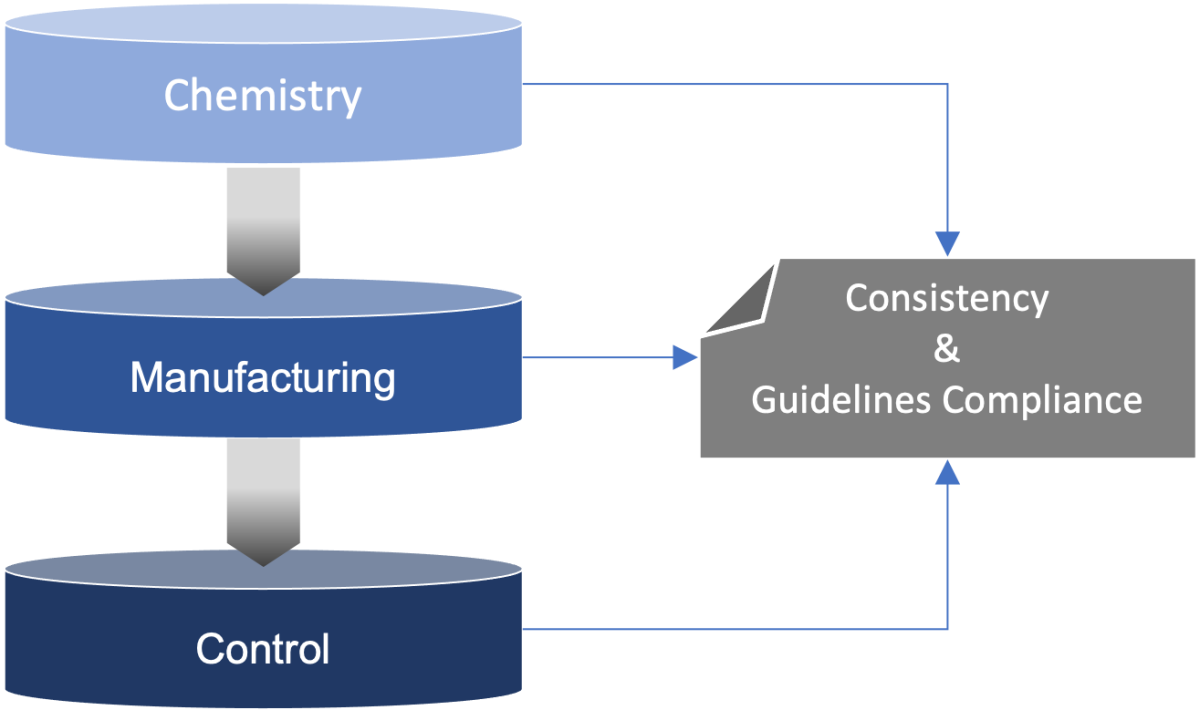
CMC Monitoring
i. Audit of the production processes (Substance & Product)
ii. Prediction of side reactions/impurities and substance stability
iii. Corrective actions
iv. Analytical Methods : validation monitoring
v. Protocol design and writing
To assess the scientific and technical maturity of your project, Pharmeb can provide you with the essential bibliographic datato strengthen your development strategy.
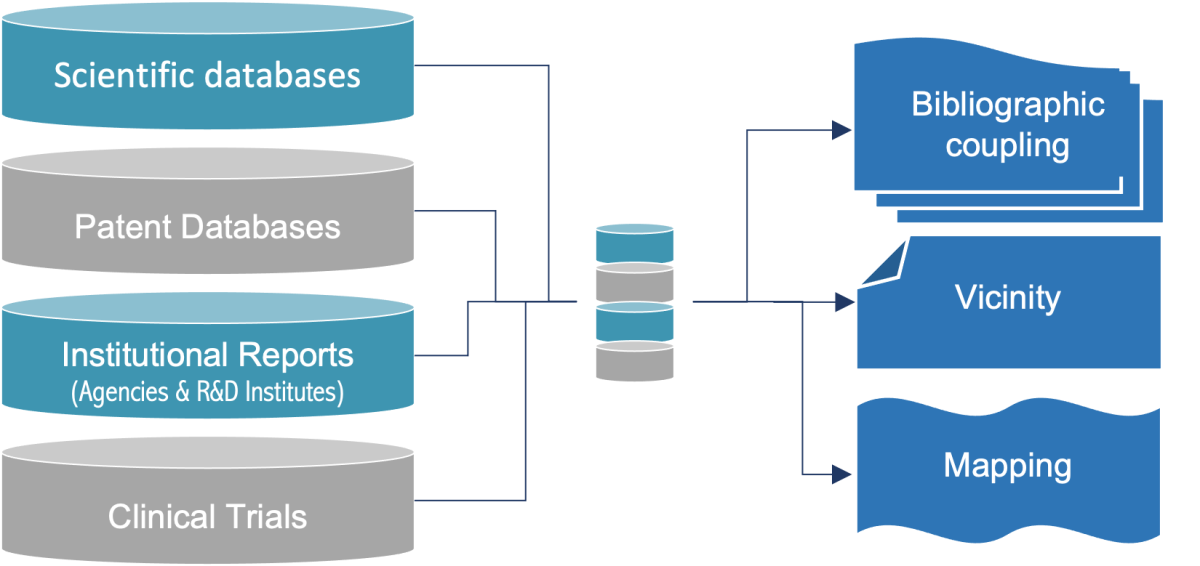
Building your project on solid ground: Bibliographic Reports & Bibliographic watch
i. Well designed bibliographic study ensures that your strategy is based on reliable data.
ii. Focused bibliographic data give you a competitive edge:
- identification of emerging trends,
- data-driven decisions.
iii. In-depth bibliographic studies help to remove technical and/or scientific obstacles during the CMC development of your pharmaceutical substances/products.
